The term saturated solution is defined in chemistry as a solution in which no more solute can be dissolved in the solvent. The solution is saturated when any additional substance results in a solid precipitate or is let off as a gas. Keep reading for a better understanding of saturated solutions and for everyday saturated solution examples.
Understanding Saturated Solutions
So what is a saturated solution? Think of the word saturated as meaning "full" — the solution is full of a solute and cannot dissolve any more of it. There are many different factors that can affect whether a solution is a saturated solution.
Elements that affect a solution's saturation include:
- the solution's temperature (warmer solution is more soluble)
- the solution's pressure
- the chemical makeup of substances involved
- the concentration and amount of solute
You can create a saturated solution by:
- adding solute to liquid until dissolving stops
- evaporating a solvent from a solution until the solute begins to crystallize or precipitate
- adding seed crystals to a solution that is supersaturated
Once the solute stops dissolving, the solution is saturated. At that point, you are creating a heterogeneous mixture instead of a homogenous solution.
Everyday Examples of Saturated Solutions
There are saturated solution examples all around you! Take a walk through your kitchen, bathroom or backyard to find saturated solutions in your everyday life.
Saturated Solution Examples in the Home
Have you ever added too much chocolate powder to your chocolate milk? No matter how hard you stir, that extra chocolate batter typically ends up at the bottom of your cup. That's because the chocolate milk was already saturated. Check out more saturated solutions that you might find at home.
- carbonated water - soda and soda water are saturated with carbon, so they give off extra carbon bubbles
- powdered juice - adding flavored sugar to water until it no longer dissolves creates a saturated solution
- soapy water - mixing powdered soap into water until it will not dissolve creates a saturated solution
- chocolate milk - chocolate powder added to milk can create saturation at the point that no more powder can be added
- bathing salts - water mixed with bathing salt becomes saturated water when there is no more ability to dissolve additional salts
- protein drinks - protein powder could be used to create a saturated solution with milk, tea or water
- sweetened beverages - sugar could be mixed into tea or coffee to the point that the beverage is saturated
- pancake syrup - once pancake syrup is saturated, all additional sugar will end up at the bottom of the bottle
- cleaning solution - mixing cleaning powder and water creates an effective cleaning solution, but once the solution is saturated, additional cleaning powder will sink to the bottom
Things that are insoluble in water cannot create the saturated solutions. For example, pepper and sand cannot be dissolved in water and therefore cannot create a saturated solution.
Outdoor Saturated Solution Examples
Like all elements of nature, outdoor solutions tend to find balance in their natural state. Once they are saturated, additional solutes don't affect that balance.
A few examples of saturated solutions in nature are:
- seawater - seawater is already saturated with salt; additional salt forms solid salt crystals instead of dissolving
- soil - the Earth's soil is saturated with nitrogen
- freshwater - most elements and metals, including potassium, can saturate freshwater
- air - the air we breathe is saturated with moisture; when there is excess moisture, it becomes dew or mist
The outdoor temperature can affect the solubility of these solutions. Warmer weather makes them more soluble, while colder weather slows solubility down.
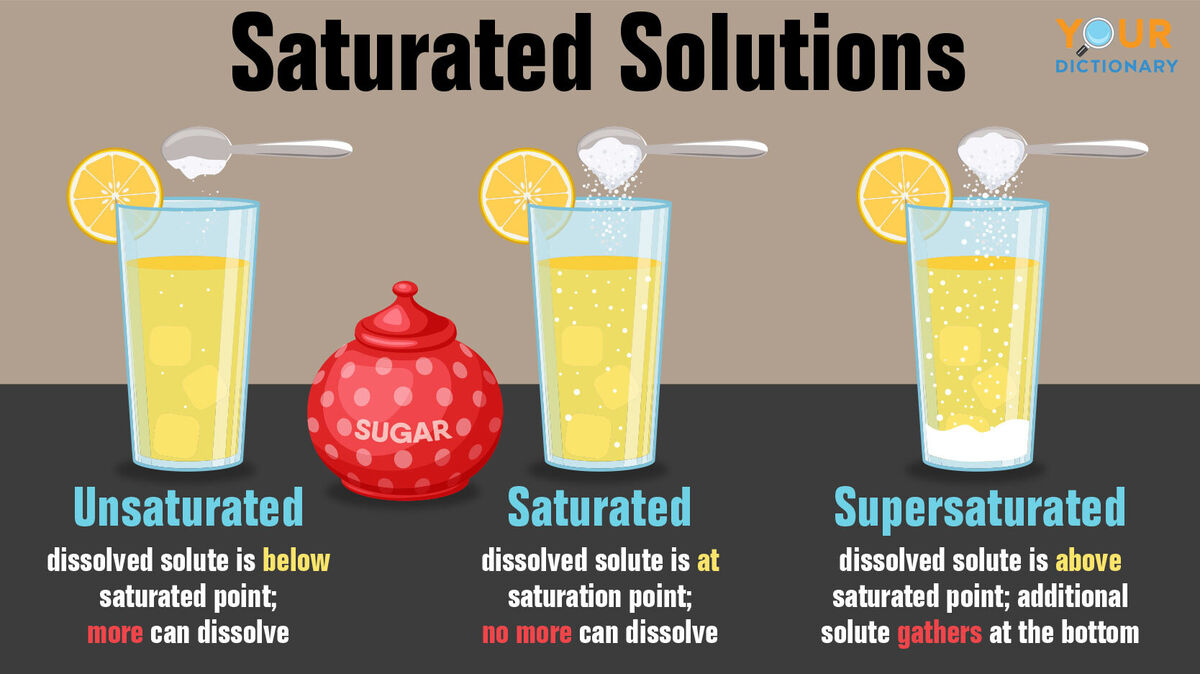
Unsaturated Solutions and Supersaturated Solutions
Solutions that aren't saturated are either unsaturated solutions or supersaturated solutions. These solutions can be defined in the following ways:
- unsaturated solutions - dissolved solute is below the saturation point (for example, water with just a pinch of salt or coffee with only one packet of sugar)
- supersaturated solutions - dissolved solute is more than the saturation point (for example, water with a cup of salt added or coffee with ten packets of sugar)
A bottle of soda is a good way to visualize unsaturated, saturated and supersaturation solutions. Before the bottle of soda is opened, it's supersaturated with carbon dioxide. When you open the bottle, the excess carbon dioxide escapes with bubbles and gas, making the solution saturated. Once the soda has gone flat and uncarbonated, it's unsaturated with carbon dioxide.
Saturate Your Chemistry Knowledge
Saturated solutions are present in our everyday lives, as well as in the chemistry lab. The next time you find chocolate milk powder at the bottom of your cup, you'll know that you've created a supersaturated solution instead of a saturated solution! Learn more about the properties that make up these solutions with these examples of chemical properties.