Heat and temperature are not the same thing. Mind blown, right? Actually, heat is a form of energy, while temperature is how hot or cold something is. While this might be the greatest difference between heat and temperature, it’s one of many. Explore the fascinating differences between heat and temperature by looking at each term individually.
What Is Heat?
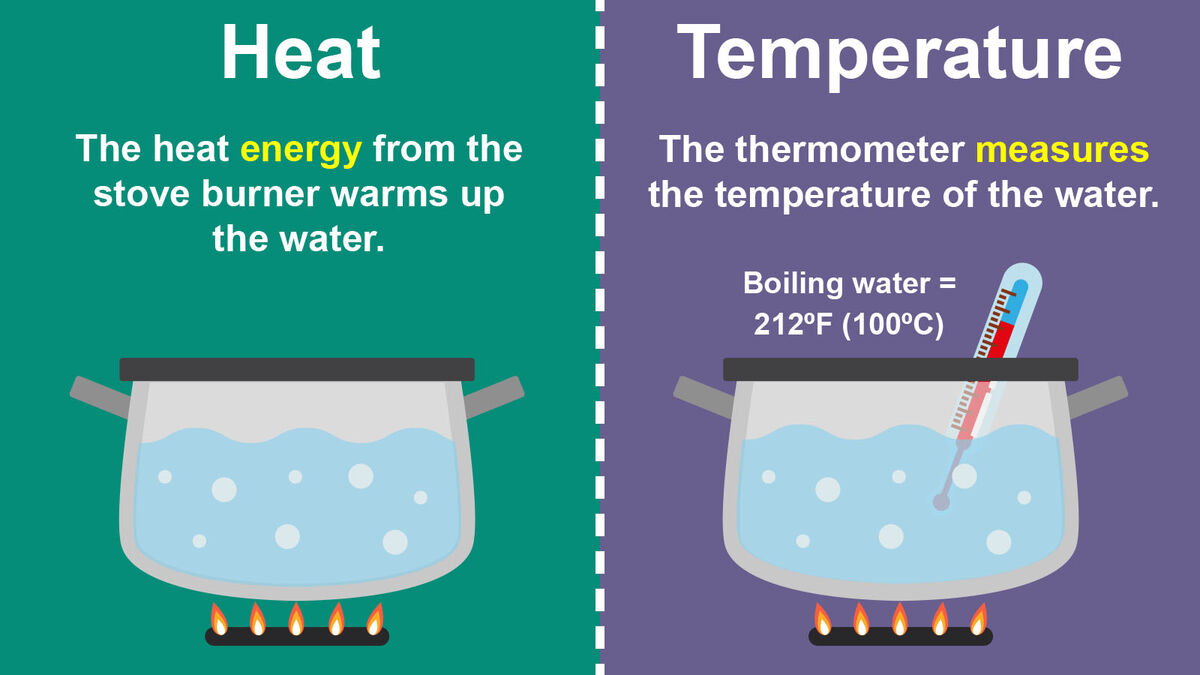
When you put water on a stove and turn on the burner, it will boil if heated to the right temperature. The heat energy the burner creates to warm up the water is called heat. By definition, heat is a form of energy where energy moves from a hot area to a colder area. In science, you might also hear the definition of heat as the total kinetic energy of an object or particle. While scientists like to get all sciency in their explanations, they are basically talking about how heat moves.
Heat Energy Example
To understand, you need to explore what happens at the molecular level. When you put a pot of water on the stove and turn on the burner, the molecules within the water start to get excited and move faster. You can even see this excitement if you’ve ever witnessed water rolling around in the pan. This is because the heat from the burner went into the colder water. Eventually, the hot water will evaporate into the colder air.
And there you have it, that’s an example of heat energy. In total, there are three different ways heat energy can transfer, called conduction, convection, and radiation. If you’ve used a stove, convection oven, and microwave to cook food, you’ve used all three types.
Measuring Heat
Since objects can gain or lose heat, there must be a way to measure heat energy. The work required for heat to be transferred is measured in joules. While joule is the most common, you might also hear of heat measured in a calorie. This makes sense, since the tool used to measure heat in a chemical reaction is called a calorimeter.
Heat wasn’t so hard to understand, so it’s time to look at the temperature.
What Is Temperature?
When you think of temperature, you might think of the evening weather. When the meteorologist tells you how cold it is outside, they are talking about temperature. Therefore, it should come as no surprise that temperature measures how cold or hot something is. This could be the air outside or even the temperature of your boiling water. Scientists will also define temperature as the average kinetic energy of a substance. Let’s see this in action.
Measuring Temperature Example
If you need to make sure that your roasting turkey has reached the right temperature for eating, you stick a thermometer in it. The thermometer will measure the heat inside the turkey to the exact degree. This is pretty much the same technique a meteorologist or physicist might use, but their thermometers are specialized to their specific fields.
Measuring Temperature
While it’s important to know what temperature is, it’s also good to know how it's measured just in case you ever see it on a paper. Temperature can be measured in a couple of different ways depending on where you are. For example, Americans use Fahrenheit while Canadians use Celsius. In physics, temperature is measured using Kelvin.
Difference Between Heat and Temperature in Physics
To clarify the difference between heat and temperature, it can be handy to have everything clearly spelled out. Use this simple table to keep heat and temperature straight.
Heat | Temperature | |
Definition | form of energy | measurement of hotness or coldness |
Units of Measurement | joule, calorie | Celsius, Kelvin, Fahrenheit |
Values | positive | positive and negative |
Property | goes from hot to cold | rises when heated; falls when cooled |
Device | calorimeter | thermometer |
Understanding Heat vs. Temperature
When you think of heat and temperature, your brain might instantly go to how hot something is. For example, the heat of the burner on the stove. Therefore, it's easy to get these two terms confused. But now you know better.
Keep your exploration of all things science going through 12 different types of everyday energy. You might also find the law of conservation of energy interesting. Energy can be quite fascinating.